Bodies, substances, particles. Gaseous, liquid and solid states of matter

 Gas (gaseous state) Gas is a state of aggregation of a substance, characterized by very weak bonds between its constituent particles (molecules, atoms or ions), as well as their high mobility.
Gas (gaseous state) Gas is a state of aggregation of a substance, characterized by very weak bonds between its constituent particles (molecules, atoms or ions), as well as their high mobility.
 Features of gases Easy to compress. They do not have their own shape and volume. Any gases are mixed with each other in any ratio.
Features of gases Easy to compress. They do not have their own shape and volume. Any gases are mixed with each other in any ratio.

 Avogadro's number The value NA = 6.022…×1023 is called Avogadro's number. This is a universal constant for the smallest particles of any substance.
Avogadro's number The value NA = 6.022…×1023 is called Avogadro's number. This is a universal constant for the smallest particles of any substance.
 A consequence of Avogadro's law 1 mole of any gas at n. y. (760 mm Hg and 00 C) occupies a volume of 22.4 liters. Vm \u003d 22. 4 l / mol - molar volume of gases
A consequence of Avogadro's law 1 mole of any gas at n. y. (760 mm Hg and 00 C) occupies a volume of 22.4 liters. Vm \u003d 22. 4 l / mol - molar volume of gases
 The most important natural mixtures of gases Composition of air: φ(N 2) = 78%; φ(O 2) = 21%; φ(CO 2) = 0. 03 Natural gas is a mixture of hydrocarbons.
The most important natural mixtures of gases Composition of air: φ(N 2) = 78%; φ(O 2) = 21%; φ(CO 2) = 0. 03 Natural gas is a mixture of hydrocarbons.
 Obtaining hydrogen. In industry: Cracking and reforming of hydrocarbons during oil refining: C 2 H 6 (t = 10,000 C) → 2 C + 3 H 2 From natural gas. CH 4 + O 2 + 2 H 2 O → 2 CO 2 +6 H 2 O
Obtaining hydrogen. In industry: Cracking and reforming of hydrocarbons during oil refining: C 2 H 6 (t = 10,000 C) → 2 C + 3 H 2 From natural gas. CH 4 + O 2 + 2 H 2 O → 2 CO 2 +6 H 2 O
 Hydrogen H 2 In the laboratory: The effect of dilute acids on metals. To carry out such a reaction, zinc and dilute sulfuric acid are most often used: Zn + 2 HCl → Zn. Cl 2 + H 2 Interaction of calcium with water: Ca + 2 H 2 O → Ca (OH) 2 + H 2 Hydrolysis of hydrides: Ca. H 2 + 2 H 2 O → Ca (OH) 2 +2 H 2 The action of alkalis on zinc or aluminum: Zn + 2 Na. OH + 2 H 2 O Na 2 + H 2
Hydrogen H 2 In the laboratory: The effect of dilute acids on metals. To carry out such a reaction, zinc and dilute sulfuric acid are most often used: Zn + 2 HCl → Zn. Cl 2 + H 2 Interaction of calcium with water: Ca + 2 H 2 O → Ca (OH) 2 + H 2 Hydrolysis of hydrides: Ca. H 2 + 2 H 2 O → Ca (OH) 2 +2 H 2 The action of alkalis on zinc or aluminum: Zn + 2 Na. OH + 2 H 2 O Na 2 + H 2
 Properties of hydrogen The lightest gas, it is 14.5 times lighter than air. Hydrogen has the highest thermal conductivity among gaseous substances. Its thermal conductivity is about seven times higher than that of air. The hydrogen molecule is diatomic - H 2. Under normal conditions, it is a colorless, odorless and tasteless gas.
Properties of hydrogen The lightest gas, it is 14.5 times lighter than air. Hydrogen has the highest thermal conductivity among gaseous substances. Its thermal conductivity is about seven times higher than that of air. The hydrogen molecule is diatomic - H 2. Under normal conditions, it is a colorless, odorless and tasteless gas.
 Oxygen In industry: From the air. The main industrial method for obtaining oxygen is cryogenic distillation. In the laboratory: From potassium permanganate (potassium permanganate): 2 KMn. O 4 = K 2 Mn. O4 + Mn. O 2 + O 2; 2 H 2 O 2 \u003d 2 H 2 O + O 2.
Oxygen In industry: From the air. The main industrial method for obtaining oxygen is cryogenic distillation. In the laboratory: From potassium permanganate (potassium permanganate): 2 KMn. O 4 = K 2 Mn. O4 + Mn. O 2 + O 2; 2 H 2 O 2 \u003d 2 H 2 O + O 2.
 Properties of Oxygen Under normal conditions, oxygen is a colorless, tasteless and odorless gas. 1 liter of it has a mass of 1.429 g. It is slightly heavier than air. Slightly soluble in water and alcohol. Very soluble in molten silver. It is paramagnetic.
Properties of Oxygen Under normal conditions, oxygen is a colorless, tasteless and odorless gas. 1 liter of it has a mass of 1.429 g. It is slightly heavier than air. Slightly soluble in water and alcohol. Very soluble in molten silver. It is paramagnetic.
 Carbon monoxide (IV) In the laboratory: From chalk, limestone or marble: Na 2 CO 3 + 2 HCl = 2 Na. Cl + CO 2 + H 2 O Ca. CO 3 + HCl \u003d Ca. Cl 2 + CO 2 + H 2 O In nature: Photosynthesis in plants: C 6 H 12 O 6 + 6 O 2 = 6 CO 2 + 6 H 2 O
Carbon monoxide (IV) In the laboratory: From chalk, limestone or marble: Na 2 CO 3 + 2 HCl = 2 Na. Cl + CO 2 + H 2 O Ca. CO 3 + HCl \u003d Ca. Cl 2 + CO 2 + H 2 O In nature: Photosynthesis in plants: C 6 H 12 O 6 + 6 O 2 = 6 CO 2 + 6 H 2 O
 Carbon monoxide (IV) Carbon monoxide (IV) (carbon dioxide) is a colorless, odorless gas with a slightly sour taste. Heavier than air, soluble in water, with strong cooling, it crystallizes in the form of a white snow-like mass - “dry ice”. At atmospheric pressure, it does not melt, but evaporates, the sublimation temperature is -78 ° C.
Carbon monoxide (IV) Carbon monoxide (IV) (carbon dioxide) is a colorless, odorless gas with a slightly sour taste. Heavier than air, soluble in water, with strong cooling, it crystallizes in the form of a white snow-like mass - “dry ice”. At atmospheric pressure, it does not melt, but evaporates, the sublimation temperature is -78 ° C.
 Ammonia (n.a.) is a colorless gas with a pungent characteristic odor (the smell of ammonia). Ammonia is almost twice as light as air, the solubility of NH 3 in water is extremely high. Ammonia is produced in the laboratory by: Interaction of alkalis with ammonium salts: NH 4 Cl + Na. OH=Na. Cl + H 2 O + NH 3 In industry: Interaction of hydrogen and nitrogen: 3 H + N = 2 NH
Ammonia (n.a.) is a colorless gas with a pungent characteristic odor (the smell of ammonia). Ammonia is almost twice as light as air, the solubility of NH 3 in water is extremely high. Ammonia is produced in the laboratory by: Interaction of alkalis with ammonium salts: NH 4 Cl + Na. OH=Na. Cl + H 2 O + NH 3 In industry: Interaction of hydrogen and nitrogen: 3 H + N = 2 NH
 Ethylene In the laboratory: Dehydration of ethyl alcohol In industry: Cracking of petroleum products: C 4 H 10 → C 2 H 6 + C 2 H 4 ethane ethene
Ethylene In the laboratory: Dehydration of ethyl alcohol In industry: Cracking of petroleum products: C 4 H 10 → C 2 H 6 + C 2 H 4 ethane ethene
 Ethylene is a colorless gas with a slight sweet smell and a relatively high density. Ethylene burns with a luminous flame; Forms an explosive mixture with air and oxygen. Ethylene is practically insoluble in water.
Ethylene is a colorless gas with a slight sweet smell and a relatively high density. Ethylene burns with a luminous flame; Forms an explosive mixture with air and oxygen. Ethylene is practically insoluble in water.
 Receiving, collecting and recognizing gases Gas name (formula) Hydrogen (H 2) Oxygen (O 2) Carbon dioxide (CO 2) Ammonia (NH 3) Ethylene (C 2 H 4) ia about substances
Receiving, collecting and recognizing gases Gas name (formula) Hydrogen (H 2) Oxygen (O 2) Carbon dioxide (CO 2) Ammonia (NH 3) Ethylene (C 2 H 4) ia about substances
 Problems Problem #1. 13.5 grams of zinc (Zn) interact with hydrochloric acid (HCl). The volume fraction of the yield of hydrogen (H 2) is 85%. Calculate the amount of hydrogen released? Task number 2. There is a gas mixture in which the mass fractions of gas are equal (%): methane - 65, hydrogen - 35. Determine the volume fractions of gases in this mixture.
Problems Problem #1. 13.5 grams of zinc (Zn) interact with hydrochloric acid (HCl). The volume fraction of the yield of hydrogen (H 2) is 85%. Calculate the amount of hydrogen released? Task number 2. There is a gas mixture in which the mass fractions of gas are equal (%): methane - 65, hydrogen - 35. Determine the volume fractions of gases in this mixture.
 Problem number 1 1) Let's write the reaction equation for the interaction of zinc (Zn) with hydrochloric acid (HCl): Zn + 2 HCl = Zn. Cl 2 + H 2 2) n (Zn) = 13.5/65 = 0.2 (mol). 3) 1 mole of Zn displaces 1 mole of hydrogen (H 2), and 0.2 mole of Zn displaces x mole of hydrogen (H 2). We get: V theor. (H 2) \u003d 0.2 ∙ 22.4 \u003d 4.48 (l). 4) Calculate the volume of hydrogen practical by the formula: V practical. (H 2) \u003d 85 ⋅ 4.48 / 100 \u003d 3.81 (l).
Problem number 1 1) Let's write the reaction equation for the interaction of zinc (Zn) with hydrochloric acid (HCl): Zn + 2 HCl = Zn. Cl 2 + H 2 2) n (Zn) = 13.5/65 = 0.2 (mol). 3) 1 mole of Zn displaces 1 mole of hydrogen (H 2), and 0.2 mole of Zn displaces x mole of hydrogen (H 2). We get: V theor. (H 2) \u003d 0.2 ∙ 22.4 \u003d 4.48 (l). 4) Calculate the volume of hydrogen practical by the formula: V practical. (H 2) \u003d 85 ⋅ 4.48 / 100 \u003d 3.81 (l).
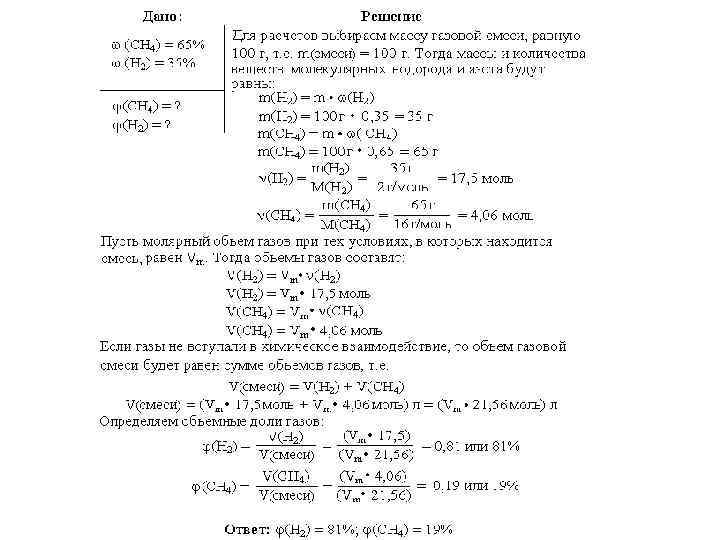
 Task number 2 There is a gas mixture, the mass fractions of gas in which are equal (%): methane - 65, hydrogen - 35. Determine the volume fractions of gases in this mixture.
Task number 2 There is a gas mixture, the mass fractions of gas in which are equal (%): methane - 65, hydrogen - 35. Determine the volume fractions of gases in this mixture.
gaseous substances.
Lecture #12
Subject:"Means acting on the central nervous system".
1. Means for anesthesia.
2. Ethyl alcohol.
3. Sleeping pills
4. Antiepileptic drugs.
5. Antiparkinsonian drugs
6. Analgesics.
Means affecting the central nervous system
Drugs for anesthesia.
Substances that cause surgical anesthesia include. Narcosis is a reversible depression of the central nervous system, which is accompanied by loss of consciousness, loss of sensitivity, a decrease in reflex excitability and muscle tone.
Means for anesthesia inhibit the transmission of nerve impulses in the synapses of the central nervous system. Synapses of the central nervous system have unequal sensitivity to narcotic substances. This explains the presence of stages in the action of drugs for anesthesia.
Stages of anesthesia:
1st stage of analgesia (stunning)
2. stage of excitation
3. stage of surgical anesthesia
1st level – superficial anesthesia
2nd level light anesthesia
3rd level deep anesthesia
4th level ultra-deep anesthesia
4. stage of awakening or agonal.
Depending on the route of administration, there are: inhaled and non-inhaled drugs.
Inhalation drugs.
Enter through the respiratory tract.
These include:
1. Volatile liquids - ether for anesthesia, halothane (halothane), chloroethyl, enflurane, isoflurane, sevoflurane.
2. gaseous substances - nitrous oxide, cyclopropane, ethylene.
It is an easily controlled anesthetic.
volatile liquids.
Ether for anesthesia- colorless, transparent, volatile liquid, explosive. Highly active. Irritates the mucous membrane of the upper respiratory tract, depresses respiration.
stages of anesthesia.
Stage 1 - stunning (analgesia). The synapses of the reticular formation are inhibited. main feature- confusion, decreased pain sensitivity, impaired conditioned reflexes, unconditioned reflexes are preserved, breathing, pulse, blood pressure are almost unchanged. At this stage, short-term operations can be performed (opening an abscess, phlegmon, etc.).
Stage 2 - excitement. The synapses of the cerebral cortex are inhibited. Inhibitory influences of the cortex on the subcortical centers are switched on, excitation processes predominate (the subcortex is disinhibited). "Rebellion of the subcortex". Consciousness is lost, motor and speech excitement (sing, swear), muscle tone increases (patients are tied). Unconditioned reflexes - cough, vomiting - intensify. Respiration and pulse are quickened, blood pressure is increased.
Complications: reflex respiratory arrest, secondary respiratory arrest: spasm of the glottis, retraction of the tongue, aspiration of vomit. This stage of the ether is very pronounced. It is impossible to operate at this stage.
Stage 3 - surgical anesthesia. Inhibition of synapses of the spinal cord. Unconditioned reflexes are inhibited, muscle tone decreases.
The operation starts at level 2, and is carried out at level 3. The pupils will be slightly dilated, almost do not react to light, the tone of the skeletal muscles is sharply reduced, blood pressure decreases, the pulse is faster, breathing is less, rare and deep.
An overdose can occur if the dosage of the narcotic substance is incorrect. And then the 4th level of super-deep anesthesia develops. The synapses of the centers of the medulla oblongata - respiratory and vasomotor - are inhibited. The pupils are wide and do not react to light, the breathing is shallow, the pulse is frequent, the blood pressure is low.
When breathing stops, the heart may still work for a while. Resuscitation begins, tk. there is a sharp depression of respiration and blood circulation. Therefore, anesthesia must be maintained at stage 3, level 3, not brought to level 4. Otherwise, the agonal stage develops. With the correct dosage of narcotic substances and the cessation of their administration develops Stage 4 - awakening. Restoration of functions goes in reverse order.
With ether anesthesia, awakening occurs in 20-40 minutes. Awakening is replaced by a long post-anesthetic sleep.
During anesthesia, the patient's body temperature decreases, metabolism is inhibited. Decreased heat production . After ether anesthesia, complications may occur: pneumonia, bronchitis (ether, irritates the respiratory tract), degeneration of parenchymal organs (liver, kidneys), reflex respiratory arrest, cardiac arrhythmias, damage to the conduction system of the heart.
Fluorothane - (halothane) - colorless, transparent, volatile liquid. Non-combustible. Stronger than ether. Mucous membranes are not irritating. The arousal stage is shorter, awakening is faster, sleep is shorter. Side effect- dilates blood vessels, lowers blood pressure, causes bradycardia (atropine is administered to prevent it).
Chloroethyl- stronger than ether, causes easily controlled anesthesia. It comes on quickly and passes quickly. Flaw- small breadth of narcotic action. It has a toxic effect on the heart and liver. Use for round anesthesia(short anesthesia when opening phlegmon, abscesses). Widely used for local anesthesia, applied to the skin. Boils at body temperature. Cools tissues, reduces pain sensitivity. Apply for superficial anesthesia during surgical operations, with myositis, neuralgia, sprains, muscles. It is impossible to overcool tissues, because. may be necrosis.
gaseous substances.
Nitrous oxide- laughing gas.
Available in pressurized bottles. Applied in a mixture with O 2. Weak drug. Combine with other narcotic substances - ether, substances for intravenous anesthesia.
Anesthesia occurs quickly, without a stage of excitation. Awakens quickly. The anesthesia is superficial. There are no side effects. Apply with injuries, myocardial infarction, transportation of patients, surgical interventions.
Cyclopropane- gas. 6 times stronger than nitrous oxide. Active. The anesthesia is easy to manage.
The stage of excitation is short, weakly expressed. Awakening immediately. There are almost no consequences. Complications- cardiac arrhythmias. Explosive.
H2O - water, Liquid metal - mercury! The liquid state is usually considered intermediate between a solid and a gas: a gas retains neither volume nor shape, while a solid retains both.
The shape of liquid bodies can be wholly or partly determined by the fact that their surface behaves like an elastic membrane. So, water can collect in drops. But the liquid is capable of flowing even under its motionless surface, and this also means non-conservation of the form (of the internal parts of the liquid body).
The molecules of a liquid do not have a definite position, but at the same time, they do not have complete freedom of movement. There is an attraction between them, strong enough to keep them close.
A substance in a liquid state exists in a certain temperature range, below which it passes into a solid state (crystallization occurs or transformation into a solid amorphous state - glass), above - into a gaseous state (evaporation occurs). The boundaries of this interval depend on the pressure.
As a rule, a substance in a liquid state has only one modification. (The most important exceptions are quantum liquids and liquid crystals.) Therefore, in most cases, a liquid is not only a state of aggregation, but also a thermodynamic phase (liquid phase).
All liquids are usually divided into pure liquids and mixtures. Some mixtures of liquids are of great importance for life: blood, sea water, etc. Liquids can act as solvents.
[edit]
Physical properties of liquids
Fluidity
Fluidity is the main property of liquids. If an external force is applied to a section of a fluid in equilibrium, then a flow of fluid particles occurs in the direction in which this force is applied: the fluid flows. Thus, under the action of unbalanced external forces, the liquid does not retain the shape and relative arrangement of the parts, and therefore takes the form of the vessel in which it is located.
Unlike plastic solids, a liquid does not have a yield point: it is enough to apply an arbitrarily small external force to make the liquid flow.
Volume Conservation
One of the characteristic properties of a liquid is that it has a certain volume (under constant external conditions). A liquid is extremely difficult to compress mechanically because, unlike a gas, there is very little free space between the molecules. The pressure exerted on a liquid enclosed in a vessel is transmitted without change to each point of the volume of this liquid (Pascal's law, also valid for gases). This feature, along with very low compressibility, is used in hydraulic machines.
Liquids typically increase in volume (expand) when heated and decrease in volume (contract) when cooled. However, there are exceptions, for example, water compresses when heated, at normal pressure and temperatures from 0 °C to approximately 4 °C.
Viscosity
In addition, liquids (like gases) are characterized by viscosity. It is defined as the ability to resist the movement of one of the parts relative to the other - that is, as internal friction.
When adjacent layers of a liquid move relative to each other, a collision of molecules inevitably occurs in addition to that due to thermal motion. There are forces that slow down the ordered movement. In this case, the kinetic energy of ordered motion is converted into thermal energy - the energy of the chaotic motion of molecules.
The liquid in the vessel, set in motion and left to itself, will gradually stop, but its temperature will rise.
The attraction and repulsion of particles determine their mutual arrangement in matter. And the properties of substances significantly depend on the location of the particles. So, looking at a transparent very hard diamond (brilliant) and soft black graphite (pencil stems are made from it), we do not guess that both substances consist of exactly the same carbon atoms. It's just that these atoms are arranged differently in graphite than in diamond.
The interaction of particles of a substance leads to the fact that it can be in three states: solid, liquid and gaseous. For example, ice, water, steam. Any substance can be in three states, but certain conditions are needed for this: pressure, temperature. For example, oxygen in the air is a gas, but when cooled below -193 °C it turns into a liquid, and at a temperature of -219 °C oxygen is a solid. Iron at normal pressure and room temperature is in a solid state. At temperatures above 1539 ° C, iron becomes liquid, and at temperatures above 3050 ° C - gaseous. Liquid mercury used in medical thermometers becomes solid when cooled below -39°C. At temperatures above 357 ° C, mercury turns into vapor (gas).
Turning metallic silver into gas, it is sprayed onto glass and get "mirror" glasses.
What are the properties of substances in different states?
Let's start with gases, in which the behavior of molecules resembles the movement of bees in a swarm. However, the bees in the swarm independently change the direction of movement and practically do not collide with each other. At the same time, for molecules in a gas, such collisions are not only inevitable, but occur almost continuously. As a result of collisions, the directions and values of the velocities of the molecules change.
The result of this motion and the lack of particle interaction in motion is that gas does not retain volume or shape, but occupies the entire volume provided to it. Each of you will consider the statements “Air occupies half the volume of the room” and “I pumped air into two-thirds of the volume of a rubber ball” as sheer absurdity. Air, like any gas, occupies the entire volume of the room and the entire volume of the ball.
What are the properties of liquids? Let's do an experiment.
Pour the water from one beaker into a beaker of another shape. The shape of the liquid has changed, but volume remains the same. The molecules did not scatter throughout the volume, as would be the case with a gas. This means that the mutual attraction of liquid molecules exists, but it does not rigidly hold neighboring molecules. They oscillate and jump from one place to another, which explains the fluidity of liquids.
The strongest is the interaction of particles in a solid. It does not allow the particles to disperse. Particles only perform chaotic oscillatory motions around certain positions. So solids retain both volume and shape. A rubber ball will retain its ball shape and volume wherever it is placed: in a jar, on a table, etc.
Water and gas. All of them differ in their properties. Liquids occupy a special place in this list. Unlike solids, molecules in liquids are not ordered. A liquid is a special state of matter that is intermediate between a gas and a solid. Substances in this form can exist only if the intervals of certain temperatures are strictly observed. Below this interval, the liquid body will turn into a solid, and above it, into a gaseous one. In this case, the boundaries of the interval directly depend on the pressure.
Water
One of the main examples of a liquid body is water. Despite belonging to this category, water can take the form of a solid or gas - depending on the ambient temperature. During the transition from a liquid to a solid state, the molecules of ordinary matter are compressed. But water behaves differently. When it freezes, its density decreases, and instead of sinking, the ice floats to the surface. Water in its usual, fluid state has all the properties of a liquid - it always has a specific volume, however, there is no definite form.
Therefore, water always retains heat under the surface of the ice. Even if the ambient temperature is -50°C, it will still be around zero under the ice. However, elementary school does not need to delve into the details of the properties of water or other substances. In grade 3, the simplest examples of liquid bodies can be given - and it is desirable to include water in this list. After all, an elementary school student should have a general idea about the properties of the world around him. At this stage, it is enough to know that water in its usual state is a liquid.
Surface tension is a property of water
Water has a greater surface tension than other liquids. Due to this property, raindrops are formed, and, consequently, the water cycle is maintained in nature. Otherwise, water vapor could not so easily turn into drops and spill onto the surface of the earth in the form of rain. Water, indeed, is an example of a liquid body, on which the possibility of the existence of living organisms on our planet directly depends.
Surface tension is due to the fact that the molecules of a liquid are attracted to each other. Each of the particles tends to surround itself with others and leave the surface of the liquid body. That is why soap bubbles and bubbles formed when water boils tend to take a liquid form - with this volume, only a ball can have a minimum surface thickness.

liquid metals
However, not only the substances familiar to man, with which he deals in everyday life, belong to the class of liquid bodies. Among this category are many different elements of the periodic system of Mendeleev. Mercury is also an example of a liquid body. This substance is widely used in the manufacture of electrical appliances, metallurgy, and the chemical industry.
Mercury is a liquid, lustrous metal that evaporates already at room temperature. It is able to dissolve silver, gold and zinc, thus forming amalgams. Mercury is an example of what are liquid bodies that are classified as dangerous to human life. Its vapors are toxic and dangerous to health. The damaging effect of mercury appears, as a rule, some time after the contact of poisoning.
A metal called cesium is also a liquid. Already at room temperature, it is in a semi-liquid form. Cesium appears to be a golden-white substance. This metal is a bit similar to gold in color, however, it is lighter.

Sulfuric acid
Almost all inorganic acids are also an example of what liquid bodies are. For example, sulfuric acid, which looks like a heavy oily liquid. It has no color or smell. When heated, it becomes a very strong oxidizing agent. In the cold, it does not interact with metals - for example, iron and aluminum. This substance shows its characteristics only in its pure form. Dilute sulfuric acid does not exhibit oxidizing properties.
Properties
What liquid bodies exist besides those listed? These are blood, oil, milk, mineral oil, alcohol. Their properties allow these substances to easily take the form of containers. Like other liquids, these substances do not lose their volume if they are poured from one vessel to another. What other properties are inherent in each of the substances in this state? Liquid bodies and their properties are well studied by physicists. Consider their main characteristics.
Fluidity
One of the most important characteristics of any body in this category is fluidity. This term refers to the ability of a body to take on a different shape, even if it is subjected to relatively weak external influences. It is thanks to this property that each liquid can be poured in jets, sprayed on the surrounding surface with drops. If bodies of this category were not fluid, it would be impossible to pour water from a bottle into a glass.
Moreover, this property is expressed in different substances to varying degrees. For example, honey changes shape very slowly compared to water. This characteristic is called viscosity. This property depends on the internal structure of the liquid body. For example, honey molecules are more like tree branches, while water molecules are more like balls with small bumps. When the liquid moves, the particles of honey seem to “cling to each other” - it is this process that gives it a greater viscosity than other types of liquids.

Shape saving
It must also be remembered that no matter what example of liquid bodies is discussed, they change only the shape, but do not change the volume. If you pour water into a beaker and pour it into another container, this characteristic will not change, although the body itself will take the form of a new vessel into which it has just been poured. The property of volume conservation is explained by the fact that both forces of mutual attraction and repulsion act between molecules. It should be noted that liquids are practically impossible to compress by means of external influence due to the fact that they always take the form of a container.

Liquid and solid bodies differ in that the latter do not obey Recall that this rule describes the behavior of all liquids and gases, and lies in their property to transfer the pressure exerted on them in all directions. However, it should be noted that those liquids that have a lower viscosity do this faster than more viscous liquid bodies. For example, if you put pressure on water or alcohol, it will spread quickly enough.

Unlike these substances, the pressure on honey or liquid oil will spread more slowly, however, just as evenly. In grade 3, examples of liquid bodies can be given without specifying their properties. Students will need more detailed knowledge in high school. However, if the student prepares additional material, this may contribute to obtaining a higher grade in the lesson.
