Examples of liquid substances. gaseous state of matter

 Gas (gaseous state) Gas is a state of aggregation of a substance, characterized by very weak bonds between its constituent particles (molecules, atoms or ions), as well as their high mobility.
Gas (gaseous state) Gas is a state of aggregation of a substance, characterized by very weak bonds between its constituent particles (molecules, atoms or ions), as well as their high mobility.
 Features of gases Easy to compress. They do not have their own shape and volume. Any gases are mixed with each other in any ratio.
Features of gases Easy to compress. They do not have their own shape and volume. Any gases are mixed with each other in any ratio.

 Avogadro's number The value NA = 6.022…×1023 is called Avogadro's number. This is a universal constant for the smallest particles of any substance.
Avogadro's number The value NA = 6.022…×1023 is called Avogadro's number. This is a universal constant for the smallest particles of any substance.
 A consequence of Avogadro's law 1 mole of any gas at n. y. (760 mm Hg and 00 C) occupies a volume of 22.4 liters. Vm \u003d 22. 4 l / mol - molar volume of gases
A consequence of Avogadro's law 1 mole of any gas at n. y. (760 mm Hg and 00 C) occupies a volume of 22.4 liters. Vm \u003d 22. 4 l / mol - molar volume of gases
 The most important natural mixtures of gases Composition of air: φ(N 2) = 78%; φ(O 2) = 21%; φ(CO 2) = 0. 03 Natural gas is a mixture of hydrocarbons.
The most important natural mixtures of gases Composition of air: φ(N 2) = 78%; φ(O 2) = 21%; φ(CO 2) = 0. 03 Natural gas is a mixture of hydrocarbons.
 Obtaining hydrogen. In industry: Cracking and reforming of hydrocarbons during oil refining: C 2 H 6 (t = 10000 C) → 2 C + 3 H 2 From natural gas. CH 4 + O 2 + 2 H 2 O → 2 CO 2 +6 H 2 O
Obtaining hydrogen. In industry: Cracking and reforming of hydrocarbons during oil refining: C 2 H 6 (t = 10000 C) → 2 C + 3 H 2 From natural gas. CH 4 + O 2 + 2 H 2 O → 2 CO 2 +6 H 2 O
 Hydrogen H 2 In the laboratory: The effect of dilute acids on metals. To carry out such a reaction, zinc and dilute sulfuric acid are most often used: Zn + 2 HCl → Zn. Cl 2 + H 2 Interaction of calcium with water: Ca + 2 H 2 O → Ca (OH) 2 + H 2 Hydrolysis of hydrides: Ca. H 2 + 2 H 2 O → Ca (OH) 2 +2 H 2 The action of alkalis on zinc or aluminum: Zn + 2 Na. OH + 2 H 2 O Na 2 + H 2
Hydrogen H 2 In the laboratory: The effect of dilute acids on metals. To carry out such a reaction, zinc and dilute sulfuric acid are most often used: Zn + 2 HCl → Zn. Cl 2 + H 2 Interaction of calcium with water: Ca + 2 H 2 O → Ca (OH) 2 + H 2 Hydrolysis of hydrides: Ca. H 2 + 2 H 2 O → Ca (OH) 2 +2 H 2 The action of alkalis on zinc or aluminum: Zn + 2 Na. OH + 2 H 2 O Na 2 + H 2
 Properties of hydrogen The lightest gas, it is 14.5 times lighter than air. Hydrogen has the highest thermal conductivity among gaseous substances. Its thermal conductivity is about seven times higher than that of air. The hydrogen molecule is diatomic - H 2. Under normal conditions, it is a colorless, odorless and tasteless gas.
Properties of hydrogen The lightest gas, it is 14.5 times lighter than air. Hydrogen has the highest thermal conductivity among gaseous substances. Its thermal conductivity is about seven times higher than that of air. The hydrogen molecule is diatomic - H 2. Under normal conditions, it is a colorless, odorless and tasteless gas.
 Oxygen In industry: From the air. The main industrial method for obtaining oxygen is cryogenic distillation. In the laboratory: From potassium permanganate (potassium permanganate): 2 KMn. O 4 = K 2 Mn. O4 + Mn. O 2 + O 2; 2 H 2 O 2 \u003d 2 H 2 O + O 2.
Oxygen In industry: From the air. The main industrial method for obtaining oxygen is cryogenic distillation. In the laboratory: From potassium permanganate (potassium permanganate): 2 KMn. O 4 = K 2 Mn. O4 + Mn. O 2 + O 2; 2 H 2 O 2 \u003d 2 H 2 O + O 2.
 Properties of Oxygen Under normal conditions, oxygen is a colorless, tasteless and odorless gas. 1 liter of it has a mass of 1.429 g. It is slightly heavier than air. Slightly soluble in water and alcohol. Very soluble in molten silver. It is paramagnetic.
Properties of Oxygen Under normal conditions, oxygen is a colorless, tasteless and odorless gas. 1 liter of it has a mass of 1.429 g. It is slightly heavier than air. Slightly soluble in water and alcohol. Very soluble in molten silver. It is paramagnetic.
 Carbon monoxide (IV) In the laboratory: From chalk, limestone or marble: Na 2 CO 3 + 2 HCl = 2 Na. Cl + CO 2 + H 2 O Ca. CO 3 + HCl \u003d Ca. Cl 2 + CO 2 + H 2 O In nature: Photosynthesis in plants: C 6 H 12 O 6 + 6 O 2 = 6 CO 2 + 6 H 2 O
Carbon monoxide (IV) In the laboratory: From chalk, limestone or marble: Na 2 CO 3 + 2 HCl = 2 Na. Cl + CO 2 + H 2 O Ca. CO 3 + HCl \u003d Ca. Cl 2 + CO 2 + H 2 O In nature: Photosynthesis in plants: C 6 H 12 O 6 + 6 O 2 = 6 CO 2 + 6 H 2 O
 Carbon monoxide (IV) Carbon monoxide (IV) (carbon dioxide) is a colorless, odorless gas with a slightly sour taste. Heavier than air, soluble in water, with strong cooling, it crystallizes in the form of a white snow-like mass - “dry ice”. At atmospheric pressure, it does not melt, but evaporates, the sublimation temperature is -78 ° C.
Carbon monoxide (IV) Carbon monoxide (IV) (carbon dioxide) is a colorless, odorless gas with a slightly sour taste. Heavier than air, soluble in water, with strong cooling, it crystallizes in the form of a white snow-like mass - “dry ice”. At atmospheric pressure, it does not melt, but evaporates, the sublimation temperature is -78 ° C.
 Ammonia (n.a.) is a colorless gas with a pungent characteristic odor (the smell of ammonia). Ammonia is almost twice as light as air, the solubility of NH 3 in water is extremely high. Ammonia is produced in the laboratory by: Interaction of alkalis with ammonium salts: NH 4 Cl + Na. OH=Na. Cl + H 2 O + NH 3 In industry: Interaction of hydrogen and nitrogen: 3 H + N = 2 NH
Ammonia (n.a.) is a colorless gas with a pungent characteristic odor (the smell of ammonia). Ammonia is almost twice as light as air, the solubility of NH 3 in water is extremely high. Ammonia is produced in the laboratory by: Interaction of alkalis with ammonium salts: NH 4 Cl + Na. OH=Na. Cl + H 2 O + NH 3 In industry: Interaction of hydrogen and nitrogen: 3 H + N = 2 NH
 Ethylene In the laboratory: Dehydration of ethyl alcohol In industry: Cracking of petroleum products: C 4 H 10 → C 2 H 6 + C 2 H 4 ethane ethene
Ethylene In the laboratory: Dehydration of ethyl alcohol In industry: Cracking of petroleum products: C 4 H 10 → C 2 H 6 + C 2 H 4 ethane ethene
 Ethylene is a colorless gas with a slight sweet smell and a relatively high density. Ethylene burns with a luminous flame; Forms an explosive mixture with air and oxygen. Ethylene is practically insoluble in water.
Ethylene is a colorless gas with a slight sweet smell and a relatively high density. Ethylene burns with a luminous flame; Forms an explosive mixture with air and oxygen. Ethylene is practically insoluble in water.
 Receiving, collecting and recognizing gases Gas name (formula) Hydrogen (H 2) Oxygen (O 2) Carbon dioxide (CO 2) Ammonia (NH 3) Ethylene (C 2 H 4) ia about substances
Receiving, collecting and recognizing gases Gas name (formula) Hydrogen (H 2) Oxygen (O 2) Carbon dioxide (CO 2) Ammonia (NH 3) Ethylene (C 2 H 4) ia about substances
 Problems Problem #1. 13.5 grams of zinc (Zn) interact with hydrochloric acid (HCl). The volume fraction of the yield of hydrogen (H 2) is 85%. Calculate the amount of hydrogen released? Task number 2. There is a gas mixture in which the mass fractions of gas are equal (%): methane - 65, hydrogen - 35. Determine the volume fractions of gases in this mixture.
Problems Problem #1. 13.5 grams of zinc (Zn) interact with hydrochloric acid (HCl). The volume fraction of the yield of hydrogen (H 2) is 85%. Calculate the amount of hydrogen released? Task number 2. There is a gas mixture in which the mass fractions of gas are equal (%): methane - 65, hydrogen - 35. Determine the volume fractions of gases in this mixture.
 Problem number 1 1) Let's write the reaction equation for the interaction of zinc (Zn) with hydrochloric acid (HCl): Zn + 2 HCl = Zn. Cl 2 + H 2 2) n (Zn) = 13.5/65 = 0.2 (mol). 3) 1 mole of Zn displaces 1 mole of hydrogen (H 2), and 0.2 mole of Zn displaces x mole of hydrogen (H 2). We get: V theor. (H 2) \u003d 0.2 ∙ 22.4 \u003d 4.48 (l). 4) Calculate the volume of hydrogen practical by the formula: V practical. (H 2) \u003d 85 ⋅ 4.48 / 100 \u003d 3.81 (l).
Problem number 1 1) Let's write the reaction equation for the interaction of zinc (Zn) with hydrochloric acid (HCl): Zn + 2 HCl = Zn. Cl 2 + H 2 2) n (Zn) = 13.5/65 = 0.2 (mol). 3) 1 mole of Zn displaces 1 mole of hydrogen (H 2), and 0.2 mole of Zn displaces x mole of hydrogen (H 2). We get: V theor. (H 2) \u003d 0.2 ∙ 22.4 \u003d 4.48 (l). 4) Calculate the volume of hydrogen practical by the formula: V practical. (H 2) \u003d 85 ⋅ 4.48 / 100 \u003d 3.81 (l).
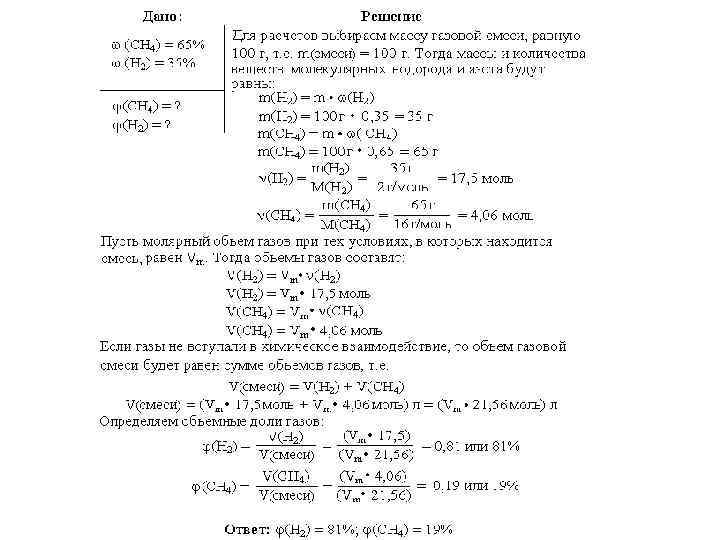
 Task number 2 There is a gas mixture, the mass fractions of gas in which are equal (%): methane - 65, hydrogen - 35. Determine the volume fractions of gases in this mixture.
Task number 2 There is a gas mixture, the mass fractions of gas in which are equal (%): methane - 65, hydrogen - 35. Determine the volume fractions of gases in this mixture.
H2O - water, Liquid metal - mercury! The liquid state is usually considered intermediate between a solid and a gas: a gas retains neither volume nor shape, while a solid retains both.
The shape of liquid bodies can be wholly or partly determined by the fact that their surface behaves like an elastic membrane. So, water can collect in drops. But the liquid is capable of flowing even under its motionless surface, and this also means non-conservation of the form (of the internal parts of the liquid body).
The molecules of a liquid do not have a definite position, but at the same time, they do not have complete freedom of movement. There is an attraction between them, strong enough to keep them close.
A substance in a liquid state exists in a certain temperature range, below which it passes into a solid state (crystallization occurs or transformation into a solid amorphous state - glass), above - into a gaseous state (evaporation occurs). The boundaries of this interval depend on the pressure.
As a rule, a substance in a liquid state has only one modification. (The most important exceptions are quantum liquids and liquid crystals.) Therefore, in most cases, a liquid is not only a state of aggregation, but also a thermodynamic phase (liquid phase).
All liquids are usually divided into pure liquids and mixtures. Some mixtures of liquids are of great importance for life: blood, sea water, etc. Liquids can act as solvents.
[edit]
Physical properties of liquids
Fluidity
Fluidity is the main property of liquids. If an external force is applied to a section of a fluid in equilibrium, then a flow of fluid particles occurs in the direction in which this force is applied: the fluid flows. Thus, under the action of unbalanced external forces, the liquid does not retain the shape and relative arrangement of the parts, and therefore takes the form of the vessel in which it is located.
Unlike plastic solids, a liquid does not have a yield point: it is enough to apply an arbitrarily small external force to make the liquid flow.
Volume Conservation
One of the characteristic properties of a liquid is that it has a certain volume (under constant external conditions). A liquid is extremely difficult to compress mechanically because, unlike a gas, there is very little free space between the molecules. The pressure exerted on a liquid enclosed in a vessel is transmitted without change to each point of the volume of this liquid (Pascal's law, also valid for gases). This feature, along with very low compressibility, is used in hydraulic machines.
Liquids typically increase in volume (expand) when heated and decrease in volume (contract) when cooled. However, there are exceptions, for example, water compresses when heated, at normal pressure and temperatures from 0 °C to approximately 4 °C.
Viscosity
In addition, liquids (like gases) are characterized by viscosity. It is defined as the ability to resist the movement of one of the parts relative to the other - that is, as internal friction.
When adjacent layers of a liquid move relative to each other, a collision of molecules inevitably occurs in addition to that due to thermal motion. There are forces that slow down the ordered movement. In this case, the kinetic energy of ordered motion is converted into thermal energy - the energy of the chaotic motion of molecules.
The liquid in the vessel, set in motion and left to itself, will gradually stop, but its temperature will rise.
The attraction and repulsion of particles determine their mutual arrangement in matter. And the properties of substances significantly depend on the location of the particles. So, looking at a transparent very hard diamond (brilliant) and soft black graphite (pencil stems are made from it), we do not guess that both substances consist of exactly the same carbon atoms. It's just that these atoms are arranged differently in graphite than in diamond.
The interaction of particles of a substance leads to the fact that it can be in three states: solid, liquid and gaseous. For example, ice, water, steam. Any substance can be in three states, but certain conditions are needed for this: pressure, temperature. For example, oxygen in the air is a gas, but when cooled below -193 °C it turns into a liquid, and at a temperature of -219 °C oxygen is a solid. Iron at normal pressure and room temperature is in a solid state. At temperatures above 1539 ° C, iron becomes liquid, and at temperatures above 3050 ° C - gaseous. Liquid mercury used in medical thermometers becomes solid when cooled below -39°C. At temperatures above 357 ° C, mercury turns into vapor (gas).
Turning metallic silver into gas, it is sprayed onto glass and get "mirror" glasses.
What are the properties of substances in different states?
Let's start with gases, in which the behavior of molecules resembles the movement of bees in a swarm. However, the bees in the swarm independently change the direction of movement and practically do not collide with each other. At the same time, for molecules in a gas, such collisions are not only inevitable, but occur almost continuously. As a result of collisions, the directions and values of the velocities of the molecules change.
The result of this motion and the lack of particle interaction in motion is that gas does not retain volume or shape, but occupies the entire volume provided to it. Each of you will consider the following statements as sheer absurdity: "Air occupies half the volume of the room" and "I pumped air into two-thirds of the volume of a rubber ball." Air, like any gas, occupies the entire volume of the room and the entire volume of the ball.
What are the properties of liquids? Let's do an experiment.
Pour the water from one beaker into a beaker of another shape. The shape of the liquid has changed, but volume remains the same. The molecules did not scatter throughout the volume, as would be the case with a gas. This means that the mutual attraction of liquid molecules exists, but it does not rigidly hold neighboring molecules. They oscillate and jump from one place to another, which explains the fluidity of liquids.
The strongest is the interaction of particles in a solid. It does not allow the particles to disperse. Particles only perform chaotic oscillatory motions around certain positions. So solids retain both volume and shape. A rubber ball will retain its ball shape and volume wherever it is placed: in a jar, on a table, etc.
Lesson type: combined
Target
- the formation of a holistic picture of the world and the awareness of a person's place in it on the basis of the unity of rational-scientific knowledge and the child's emotional-valuable understanding of personal experience of communication with people and nature;
Problem:
what is a body, substance, particle?
Tasks:
Distinguish between bodies, substances and particles,
Conduct experiments using laboratory equipment
Subject Results
will learn
To characterize the concepts of "body", "substance", "particles";
Distinguish between bodies and substances, classify them.
Universally Learning Actions (UUD)
Regulatory: adequately use speech to plan and regulate their activities; to transform a practical task into a cognitive one.
Cognitive: pose and formulate problems, control and evaluate the process and result of activities (experience); transfer of information.
Communicative: cost a monologue statement, argue your position.
Personal Outcomes
Motivation for learning activities
Basic concepts and definitions
Bodies, substances, particles. natural and artificial bodies. Solid, liquid, gaseous substances
Checking readiness for mastering new material
Remember what groups you can divide all the objects that surround us.
Consider the diagram. What two groups can bodies be divided into? Give examples of the bodies of each group.
Learning new material
Any object, any living being can be called a body. Stone, lump of sugar, wood, bird, wire are bodies. It is impossible to list all the bodies, there are countless of them. The sun, the planets, the moon are also bodies. They are called celestial bodies
SUBSTANCES
Bodies are made up of substances. A lump of sugar is a body, and sugar itself is a substance. Aluminum wire is the body, aluminum is the substance.
There are bodies that are formed not by one, but by several or many substances. Living bodies have a very complex composition. For example, plants contain water, sugar, starch and other substances. Many different substances are formed and the bodies of animals, humans
So, substances are what bodies are made of.
Distinguish solid, liquid and gaseous substances. Sugar, aluminum are examples of solids. Water is a liquid substance. Air consists of several gaseous substances (gases).
bodyandsubstances
body. Substances
Experience. Fromwhatconsistsubstances
Threestatessubstances
PARTICLES
Experience. Let us take a body formed by one substance, a piece of sugar. Dip it in a glass of water, stir. At first, sugar is clearly visible, but gradually becomes invisible. Let's taste the liquid. She is sweet. This means that the sugar has not disappeared, it has remained in the glass. Why don't we see it? Make a guess.
A piece of sugar broke up into tiny, invisible particles, of which it consisted (dissolved), and these particles mixed with water particles.
Conclusion: experience proves that substances, and hence bodies, are composed of particles.
Each substance consists of special particles, which differ in size and shape from the particles of other substances.
Scientists have found that there are gaps between the particles. In solids, these gaps are very small, in liquids they are larger, in gases even more. In any substance, all particles are constantly moving.


Comprehension and understanding of the acquired knowledge
Presentation "Bodies, substances, molecules"
bodyandsubstancesaroundus
1.Check with the textbook if the following statements are true.
Any object, any living being can be called a body.
Substances are what bodies are made of.
2. Select from the list first bodies, then substances. Test yourself on the Self Test Pages.
Horseshoe, glass, iron, brick, sugar, watermelon, salt, starch, stone.
3. Use the model to show the process of dissolving a piece of sugar in water.
4. Use models to depict the location of particles in solid, liquid, gaseous substances.
Independent application of knowledge
What are called bodies? Give examples.
What are substances? Give examples. 3. What are substances made of? How to prove it? 4. What can you tell about particles?
Homework. Write in the dictionary: body, substance, particle.
Information sources:
A. A. Pleshakov textbook, workbook The world around Grade 3 Moscow
"Enlightenment" 2014
Presentation Hosting the world
Back forward
Attention! The slide preview is for informational purposes only and may not represent the full extent of the presentation. If you are interested in this work, please download the full version.
Back forward
Back forward
Age: Grade 3
Subject: Bodies, substances, particles.
Lesson type: learning new material.
Lesson duration: 45 minutes.
Lesson Objectives: to form the concept of a body, substance, particle, to teach to distinguish substances according to their characteristics and properties.
Tasks:
- To acquaint children with the concepts of body, substance, particle.
- Learn to distinguish between substances in different states of aggregation.
- Develop memory, thinking.
- Improve self-esteem and self-control skills.
- Increase the psychological comfort of the lesson, relieve muscle tension (dynamic pauses, change of activity).
- Build friendships within the team.
- Cultivate an interest in the environment.
Equipment:
1. Multimedia interactive presentation (Appendix 1). Presentation management Appendix 2
2. Drawings (solid, liquid, gaseous substances).
3. Metal ruler, rubber ball, wooden cube (at the teacher).
4. For the experiment: a glass, a teaspoon, a sugar cube; boiled water (on the tables for children).
During the classes
I. Organizational moment.
The teacher greets the children, checks the readiness for the lesson, addressing the students: “Today you will do all the tasks in groups. Let's repeat the rules of working in a group ”(slide No. 2).
- Treatment of comrades - "politeness";
- The opinion of others - “learn to listen, prove your point of view”;
- Working with sources of information (with a dictionary, a book) - highlight the main thing.
II. Learning new material.
Setting a learning goal: today we are starting to study the topic “This amazing nature” - we will take a virtual tour (slide No. 3). On the slide: a drop of water, a sugar bowl (storage container), a hammer, a wave (water), clay, metal.
The teacher asks the question “Did all the words make it possible to accurately represent the subject?”
Those words that accurately help to represent the subject, namely, have outlines, shape, are called bodies. What these objects are made of is called substances.
Working with the source of information (dictionary of S.I. Ozhegov):
Write the definition in a notebook: “Those objects that surround us are called bodies” (slide number 4).
Slide number 5. The teacher invites students to compare the pictures on the slide: a rubber ball, an envelope, a wooden cube.
Task 1: find common. All bodies have a size, shape, etc.
Task 2: identify the main features of bodies. Answer on slide 6: control button “answer 2”.
Slide number 6. Pictures are triggers. The ball is round, rubber, bright. Envelope - rectangular, paper, white. Cube - wooden, large, beige.
Together with the guys we conclude “Every body has a size, shape, color”. We write in a notebook.
Slide number 7. What is nature? Choose the correct answer from the three options:
Slide number 8 - work with cards. Students have cards with images of bodies (objects) on their tables. Let's invite students to divide the cards into two groups: table, sun, tree, pencil, cloud, stone, books, chair. Write down the answers in your notebook. We ask students to read the names of the bodies, this will be 1 group. On what basis did they place the words in this group? We do the same with the second group.
Correct answer:
We draw a conclusion. How did we divide the words (by what principle?): there are bodies that are created by nature, and there are those that are created by human hands.
We draw up a block in a notebook (Figure 1).

Slide number 9. Reception "Interactive tape". The slide shows natural and artificial bodies. Using the scroll button, which is also a trigger, we view natural and artificial bodies (each time pressing the button changes the grouped pictures).
We consolidate the knowledge gained with the help of the game “Traffic Light” (slides 10-12). The game is to find the correct answer.
Slide 10. Task: find natural bodies. Of the proposed bodies on the slide, you must select only natural bodies. The picture is a trigger - when pressed, a traffic light (red or green) appears. The sound files help students to make sure they choose the right answer.
Teacher: Let's remember what we talked about at the beginning. We found it difficult to determine exactly whether metal, water, clay are bodies and came to the conclusion that they do not have exact outlines, shapes, and therefore are not bodies. We call these words substances. All bodies are made up of matter. Write down the definition in your notebook.
Slide 13. On this slide, we will consider two examples.
Example 1: scissors are a body, what they are made of is a substance (iron).
Example 2: water drops - bodies, the substance of which the drops are composed - water.
Slide number 14. Consider bodies that consist of several substances. For example, a pencil and a magnifying glass. On the slide, we separately look at the substances that make up the pencil. To demonstrate, we press the control buttons: “graphite”, “rubber”, “wood”. In order to remove unnecessary information, click the cross.
Consider what substances the magnifying glass consists of. We press the triggers “glass”, “wood”, “metal”.
Slide number 15. To consolidate, consider two more examples. What is a hammer made of? The hammer is made of iron and wood (handle). What are knives made of? Knives are made of iron and wood.
Slide number 16. Consider two objects that consist of several substances. Meat grinder: made of iron and wood. Sledge: made of iron and wood.
Slide 17. We conclude: bodies can consist of one substance, or they can consist of several.
Slides 18, 19, 20. Reception "Interactive tape". Demonstrate to students. One substance can be part of several bodies.
Slide 18. Substances are wholly or partially composed of glass.
Slide 19. Substances are completely or partially composed of their metal.
Slide 20. Substances wholly or partly consist of plastics.
Slide 21. The teacher asks the question “Are all substances the same?”
On the slide, click the control button “Start”. Writing in a notebook: all substances are composed of the smallest invisible particles. We introduce the classification of substances according to their state of aggregation: liquid, solid, gaseous. The slide uses triggers (arrows). When you click on the arrow, you can see a picture with particles in a given state of aggregation. Pressing the arrow again will make the objects disappear.
Slide 22. Experimental part. It is necessary to prove that the particles are the smallest, invisible to the eye, but retaining the properties of matter.
Let's do an experiment. On the students' tables there are trays with a set of the simplest laboratory equipment: a glass, a stirring spoon, a napkin, a piece of sugar.
Dip a piece of sugar into a glass, stir until completely dissolved. What are we observing? The solution has become homogeneous, we no longer see a piece of sugar in a glass of water. Prove that sugar is still present in the glass. How? To taste. Sugar: white substance, sweet in taste. Conclusion: after dissolution, sugar did not cease to be sugar, because it remained sweet. This means that sugar consists of tiny particles that are not visible to the eye (molecules).
Slide 23. Consider the arrangement of particles in substances with a solid state of aggregation. We demonstrate the arrangement of particles and matter (examples) using the “interactive tape” technique - the scroll button allows you to show the pictures the required number of times. We write down the conclusion in a notebook: in solids, particles are located close to each other.
Slide 24. The location of particles in liquid substances. In liquid substances, the particles are located at some distance from each other.
Slide number 25. The location of particles in gaseous substances: the particles are located far from each other, the distance between them is much greater than the particle size itself.
Slide 31. It's time to take stock. Together with the teacher, they recall what they learned in the lesson. The teacher asks questions:
- Everything that surrounds us is called .... bodies
- Bodies are natural and artificial.
- Write the diagram in your notebook. Teacher: Let's look at the diagram. Bodies are natural and artificial, substances can be solid, liquid, gaseous. Substances are made up of particles. The particle retains the properties of the substance (recall that sugar remained sweet when dissolved). The slide uses triggers. Click on the “Body” shape, arrows appear, then shapes labeled “Artificial” and “Natural”. When you click on the “substance” figure, three arrows appear (liquid, solid, gaseous).
Slide number 30. Fill in the table. Read the instructions carefully.
(Mark with “ + ” in the appropriate column, which of the listed substances are solid, liquid, gaseous).
| Substance | Solid | Liquid | gaseous |
| Salt | |||
| Natural gas | |||
| Sugar | |||
| Water | |||
| Aluminum | |||
| Alcohol | |||
| Iron | |||
| Carbon dioxide |
Checking the progress of work (slide 30). In turn, the children name the substance and explain to which group it was assigned.
Lesson summary
1) Summing up
You worked together.
Find out which group was the most attentive in the lesson. The teacher asks the question: "What are called bodies, what characterizes the body, give an example." Students answer. Everything that surrounds us is called bodies. What are the substances according to the state of aggregation: liquid, solid, gaseous. What are substances made of? Give examples of how particles retain the properties of substances. For example, if we salt the soup, how do we know that the properties of the substance have been preserved? To taste. Fill in the diagram (Figure 2)

Discussion: what do you agree with, what do you disagree with.
What have you learned? Children report. ( Bodies are all the objects that surround us. Bodies are made up of substances. Substances - from particles).
Homework
The teacher tells the children homework (optional):
- solve a small test (Appendix 5).
- interactive test (Appendix 3).
- view a presentation about water (Annex 7). In the presentation, you can get acquainted with six well-known facts about water. Think guys, why do you need to get to know this substance better? Answer: The most common substance on Earth. And what other substance would you like to invite to your place (creating virtual tours).
- study the electronic textbook (Appendix 4).
Note: the teacher can additionally use slides No. 32, 33, 36.
Slide number 32. Task: test yourself. Find products (interactive test).
Slide number 33. Task: test yourself. Find the bodies of animate and inanimate nature (interactive test).
Slide number 36. Task: divide the bodies into bodies of animate and inanimate nature (interactive test).
Literature.
- Gribov P.D. how a person explores, studies, uses nature. 2-3 classes. Volgograd: Teacher, 2004.-64 p.
- Maksimova T.N. Lesson developments for the course "The World Around": Grade 2. - M.: VAKO, 2012.-336s. - (To help the school teacher).
- Reshetnikova G.N., Strelnikov N.I. The world. Grade 3: entertaining materials. - Volgograd: Teacher, 2008. - 264 p.: ill.
- Tikhomirova E.M. Tests on the subject “The world around us”: Grade 2: to the A.A. Pleshakov “The world around us. Grade 2”. - M.: Publishing house "Exam", 2011. - 22 p.
